Vaccine will adapt the rabies vaccine platform to reduce the incidence and burden of Lyme disease
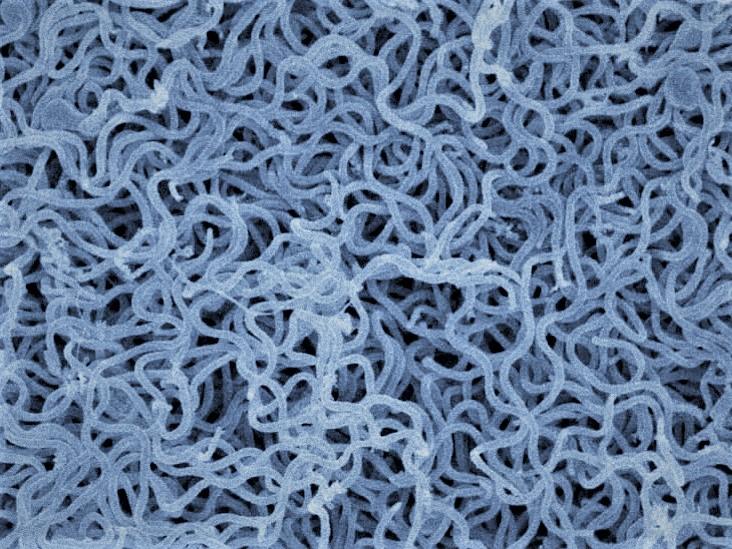
Borrelia burgdorferi, the pathogen causing Lyme disease, in a group of cells under a transmission electron microscope
Image Credit: Utpal Pal's lab
In the heart of tick season and with COVID-19’s affinity for those who are immunocompromised, contracting Lyme disease is particularly concerning now and in the coming years. Addressing the need for an effective Lyme disease vaccine to prevent the upwards of 300,000 new cases in the U.S. alone each year and reduce the burden of chronic or post-treatment Lyme disease, the University of Maryland (UMD) is leading a new $3.5 million grant from the National Institutes of Health (NIH) to develop a novel “next-generation” Lyme disease vaccine. Collaborating with Thomas Jefferson University’s Jefferson Vaccine Center, Utpal Pal, professor in Veterinary Medicine at UMD, will use his expertise as a renowned tick immunobiologist to adapt the safe and effective rabies vaccination platform to produce antibodies that can provide protection against Borrelia burgdorferi, the bacteria responsible for Lyme disease, with the hope of applying this work to other tick-borne diseases in the future.
“Borrelia infection is treatable with antibiotics, but it is often misdiagnosed, and you have a substantial fraction of antibiotic-treated Lyme patients that develop a variety of neurological symptoms called post-treatment Lyme Disease Syndrome. There is no cure for that, no drug, and no treatment. The only way to prevent this is to prevent Lyme, and a vaccine is a good answer for that,” says Pal.
As an expert in Borrelia and tick-borne disease, Pal is partnering with Matthias Schnell at Thomas Jefferson University, director of the Jefferson Vaccine Center known for the study and application of the rabies virus as a platform for vaccination. “We are using the rabies virus as a delivery platform to send in some vaccine candidates for Borrelia,” explains Pal. “For rabies, we can produce an inactivated virus that helps the body produce the antibodies needed to fight it. Since we can produce the rabies vaccine and antibody proteins safely, why not have this virus produce other types of proteins that can do something else, like fight Borrelia?”
This is an approach that Schnell and colleagues have studied and proven effective for other viral vaccinations, but it has not been previously explored for Borrelia and tick-borne diseases. Using proteins that Pal’s lab previously identified as vaccine candidates, Pal and his team hope to combine these proteins with the rabies virus to deliver long-lasting, safe, and effective immunity. This includes testing not only the four candidate proteins, but also three major types of rabies vaccine platforms that could be effective for Lyme disease.
“Viral vaccines are produced three different ways,” explains Pal. “The first is live attenuated virus, meaning that the virus is whole and viable, but you can make it incapable of producing full blown infection. The second type of vaccine, which is more popular for safety and efficacy and is the platform for the actual rabies vaccine, is an inactivated virus. It’s the whole virus, but it is inactivated and dead. And for the third type of vaccine, we take a protein from the virus or just a piece of the virus and produce a virus-like particle or VLP. In this case, you create a sort of shell of a virus that looks like the virus and has viral proteins on the outside but no actual virus on the inside, so the body is tricked into developing defenses against the virus itself.”
In their novel approach to developing a Lyme disease vaccine, Pal and Schnell will be examining all three of these possible platforms, using the rabies virus itself as the ultimate delivery method.
“Each vaccine has two parts,” says Pal. “One is actually the target protein or the antigen, and the other is how you deliver this to the human. This is important. If you take a protein and you inject it into the animal, there will be nothing, the protein will just disappear into the bloodstream. That is why vaccines use adjuvant chemicals, a compound that makes the antigen stable so the immune system can recognize it, see it, and mount an immune response. The best adjuvants aren’t safe for humans. But if we use the virus platform, we don’t need adjuvant because the virus acts like an adjuvant and often produces a strong immune response. So we are using the virus’ ability to produce a strong immune response to supply the Borrelia proteins and produce the appropriate antibodies.”
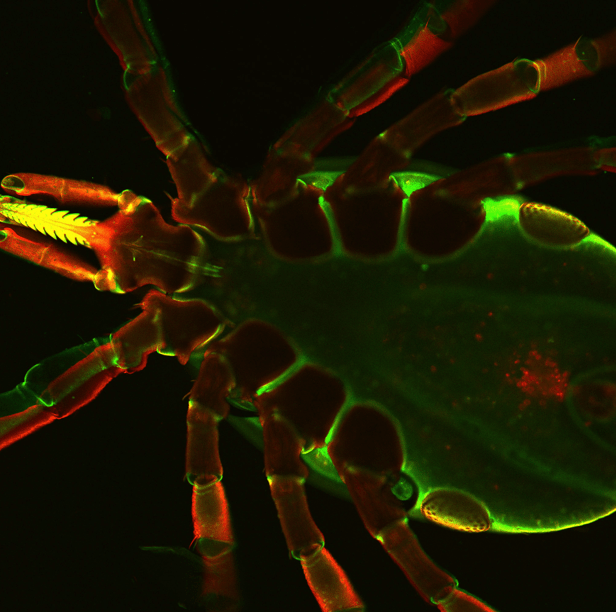
In addition to this unique approach, Pal is also looking at stopping Borrelia infection from two different sides, targeting both Borrelia proteins and the tick proteins that actually help keep the bacteria alive and ensure it can be transferred to humans. “It is like we are trying to dismantle the train [Borrelia] and the tracks [the delivery mechanism from ticks to humans] at the same time, which has never been done before,” says Pal. “Tick proteins are helping Borrelia, and with the vaccine, we would like to develop antibodies so that when the tick starts to feed, those antibodies will bind to tick targets as well as Borrelia and try to prevent the infection.” To do this, Pal will target proteins previously identified by his lab, including two Borrelia proteins published in The Journal of Infectious Diseases (BB0405 and BBI39) and two parts of a tick protein published in The Journal of Biological Chemistry.
Once the best combination of vaccine platform and protein antigens is identified, Pal and his team will test this option in mice to examine the underlying protection mechanisms of the vaccine and determine its overall effectiveness.
“This is an exciting project because we are able to repurpose the very effective rabies virus delivery platform to create something new,” says Pal. “Lyme disease is a very prevalent infection, but with a cure that doesn’t work for everyone. These chronic cases create long-term problems for diagnosis and treatment, and those patients are very difficult to manage. If we can produce something effective for Lyme disease, we can help with this problem and also understand how we can apply it to other tick-borne diseases.”
This work is funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), Project Number 1R01AI154542-01.