Expertise
- Spotted Fever Rickettsia
- Intracellular bacteria
- Innate Immunology
- Vaccination
- Host-pathogen Interactions
About the Lab
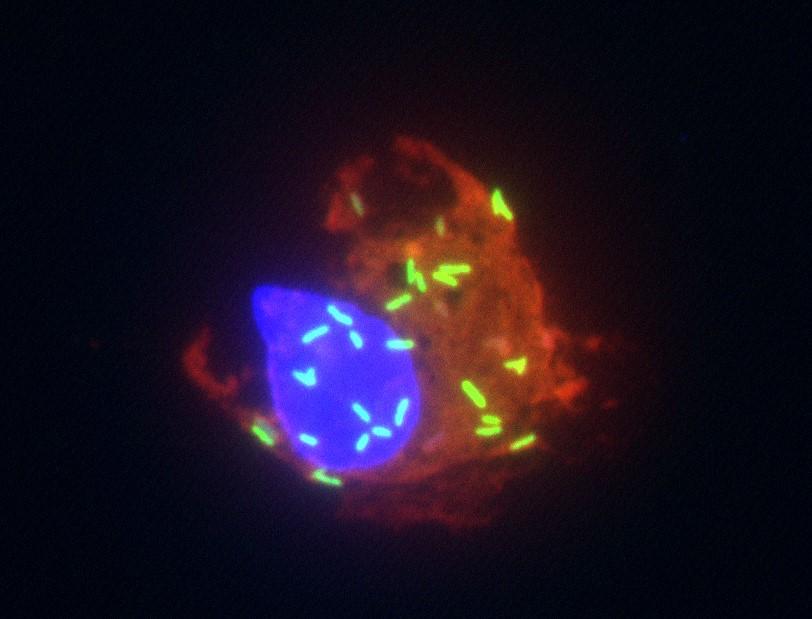
We study nasty little bacteria called Rickettsia and how these bacteria interact with mammal hosts. Rickettsia (green in the picture) are obligate intracellular bacteria that must live inside of a nucleated host cel (red and blue in the picture). Unfortunately for us, the most dangerous Rickettsia species love to grow within the cells that line our blood vessels. After being transmitted to a human by an infected tick, the bacteria grow within blood vessels, and eventually the combination of Rickettsia growth and our own immune responses lead to loss of vascular function. This is how we come up with the name of these infections: Spotted Fever Rickettsia infections.
The most well known and dangerous Rickettsia in the United States is Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever. Our lab studies how R. rickettsii and other rickettsial pathogens can hijack our own cells to grow and, conversely, how our immune system can effectively fight Rickettsia infections. By performing this fundamental research we hope to identify aspects of Rickettsia biology or mammalian immunity that can be harnessed for diagnostic, antibiotic, and vaccine production.
We are located in the Department of Veterinary Medicine at the University of Maryland-College Park. The Department of Veterinary Medicine, University of Maryland, College Park and the Maryland Campus of the Virginia-Maryland College of Veterinary Medicine (VMCVM) are integrated as a single unit housed in the Avrum Gudelsky Veterinary Center at the University of Maryland, College Park Campus.
Projects
Host-Directed Therapeutics
We have identified a series of drugs that are effective at controlling Rickettsia infection in culture. The cool parts are these drugs are 1) already approved by the FDA and 2) do not target the bacteria. Each of these drugs actually target the host cell so that the intracellular environment is no longer hospitable to bacteria. We aim to define the potential for using these drugs against multiple different infections.
Rickettsia vs. Innate Immunity
Bacteria and their hosts have had millennia of evolution to form interactions that we observe as disease or commensalism or everything in between. Rickettsia and mammals have developed a back-and-forth interplay with a portion of the immune system called the complement system. Rickettsia have evolved mechanisms to steal proteins that normally coat our own cells to protect us from the immune system. However, this has a cost. The complement system cannot kill the bacteria, but is does "call for help" from other portions of the immune system to ultimately fight the infection. We aim to further define this interplay to determine if we develop new methods to fight infection.
Vaccinology
Though Rickettsia infections have historically been major killers, there are not available vaccines to prevent these infections. We are always searching for new and better vaccine targets on the bacterial surface that may lead to developing new ways to fight Rickettsia infections.
Defining Changes to the Bacteria in Different Hosts
The Rickettsia life cycle involves repeated cycles of infection in mammalian and arthropod (ticks, lice, fleas) hosts. The environment within these arthropods is drastically different than mammalian hosts. Vector-borne bacteria have to adapt to each host type by changing nearly everything about their biology. We aim to better define these changes to Rickettsia RNA and proteins that contribute to survival in each host type.
People
Sean P Riley
Assistant Professor
Postdoc, Louisiana State University
Postdoc, University of Chicago
Ph.D, University of Kentucky
B.S. University of Illinois, Urbana/Champaign
Mustapha (Masten) Dahmani
Postdoctoral Scholar
Ph.D. Aix-Marseille University, France
MSc. Aix-Marseille University, France
DVM, Veterinary High School, Algera
Jinyi (Cornelia) Zhu
PhD Candidate- Biological Sciences
B.S- University of Maryland
Marissa Elassal
Undergraduate Student- General Biology
Publications
Dahmani M, Zhu JC, Cook JH, Riley SP. Anaphylatoxin signaling activates macrophages to control intracellular Rickettsia proliferation. Microbiol Spectr. 2023 Dec 12;11(6):e0253823. doi: 10.1128/spectrum.02538-23. Epub 2023 Oct 19. PubMed PMID: 37855623; PubMed Central PMCID: PMC10714731.
Snyder AM, Riley SP, Robison CI, Karcher DM, Wickware CL, Johnson TA, Weimer SL. Behavior and Immune Response of Conventional and Slow-Growing Broilers to Salmonella Typhimurium. Front Physiol. 2022;13:890848. doi: 10.3389/fphys.2022.890848. eCollection 2022. PubMed PMID: 35586720; PubMed Central PMCID: PMC9108930.
Dahmani M, Cook JH, Zhu JC, Riley SP. Contribution of classical complement activation and IgM to the control of Rickettsia infection. Mol Microbiol. 2021 Dec;116(6):1476-1488. doi: 10.1111/mmi.14839. Epub 2021 Nov 13. PubMed PMID: 34725868; PubMed Central PMCID: PMC8955150.
Esteves E, Fongsaran C, Langohr IM, Riley SP, Labruna MB, Daffre S, Fogaça AC, Macaluso KR. Comparative Analysis of Infection by Rickettsia rickettsii Sheila Smith and Taiaçu Strains in a Murine Model. Pathogens. 2020 Sep 10;9(9). doi: 10.3390/pathogens9090744. PubMed PMID: 32927666; PubMed Central PMCID: PMC7557639.
Pictures