Eco-evolutionary Dynamics of Age-specific Resistance
Samuel Slowinski, Allyson Kido, Andrea Shirdon, Emily Bruns (Dept of Biology UMD, College Park)
Mature plant resistance is a well-known phenomenon in many crop species. Older plants tend to have a higher level of resistance, and better tolerance of pathogens than young seedlings. This pattern is not restricted to crop species, but also occurs in wild plants, as well as invertebrates and even vertebrates. Our lab is interested in how these patterns of age-specific resistance evolve in nature. Another way of putting this: why don’t plants ev olve higher levels of seedling resistance? Afterall, from an evolutionary perspective it is worse to get infected prior to reproduction than later in life!
olve higher levels of seedling resistance? Afterall, from an evolutionary perspective it is worse to get infected prior to reproduction than later in life!
Our lab has been using the herbaceous wild species, Silene latifolia (Caryophyllaceae) and its naturally occurring obligate anther-smut pathogen (caused by the Basidiomycete fungus: Microbotryum lychnidis-dioicae) to test several hypotheses about the evolution of age-specific disease resistance. The fungus, M. lychnidis-dioicae is highly specialized to S. latifolia. It colonizes the plant’s meristems where it grows asymptomatically until flowering. When the plant flowers, the fungus sporulates in the anthers, replacing the pollen and sterilizing the flower (Fig 1). In female flowers, the fungus induces anther production and sterilizes the ovary (Fig 1C). Transmission of the spores to mature, flowering plants is facilitated by pollinators. Spore transmission can also occur to non-flowering juvenile plants through localized wind transmission. Both the plant and fungus are native to Europe but were both introduced to the US in the 1800s and have subsequently become naturalized in eastern US. We have previously shown that seedlings are significantly more susceptible to anther-smut disease than adult, flowering plants.
Cost of resistance experiment: One hypothesis for the maintenance of juvenile susceptibility (e.g. low juvenile resistance) is that the cost of resistance is higher at the juvenile stage than at the adult stage. To test this hypothesis, we set up a common garden experiment at the MAES facility in Beltsville, to quantify fitness components of 45 families of the host plant in the absence of disease. Plants were reared for two years and scored for survival and flowering every 2 weeks (Fig 2a). These families were simultaneous reared to four ages in the greenhouse and directly challenged with spores of M. lychnidis-dioicae to determine age-specific susceptibility.

Fig 2. A) Common garden experiment at Beltsville MAES. B-C) Examples of flower number differences among families of S. latifolia in common garden.
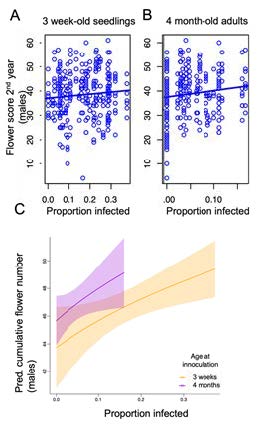
Results: We found that there was a significant trade-off between family-level disease resistance at both juvenile and adult stages, with more susceptible plants producing more flowers over the course of the season (Fig 2b). However, both juvenile and adult resistance were similarly costly (as evidenced by similar slopes; Fig 2c).
Transmission experiment: A second explanation for the maintenance of juvenile susceptibility is that the strength of natural selection for disease resistance is higher for adults than juveniles. To test this we put 175 potted S. latifolia plants inoculated with M. lychnidis-dioicae into our common garden experiment and monitored transmission to the adult plants over the course of two years. We found that floral traits such as sex and number of flowers were important predictors of infection risk in the field. Male plants produced more flowers than females are had a significantly higher probability of infection. Infection risk likely increases with flower number because pollinators are a major route of transmission to adult plants. We also found that plant families with higher adult resistance (measured in the greenhouse) had significantly lower infection rates. Taken together, our results show that the strength of natural selection on adult resistance in the field is also mediated by floral traits that affect the level of disease exposure.
Fig 3. Results from the cost of resistance experiment. Relationship between greenhouse-measured seedling (A) or adult (B) resistance and total flower production in the field in the absence of disease. Each circle is a replicate male plant in the field. Both lines have a significant, positive slope indicating that more resistant plant families on the left hand side of the graphs (low proportion infected) produce fewer flowers than highly susceptible families. C) Results of aster-life history statistical models that integrate plant survival and flowering into a cumulative measure of fitness.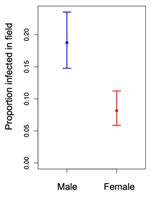
Fig 4. Proportion of male and female plants that became infected in the transmission experiment.
Significance: Age-specific resistance is a widespread phenomenon in plants, and fruitful avenue for crop improvement. However, we know very little about the genetic and ecological factors driving the evolution of this important trait in natural plant populations. Our work so far has shown that natural populations of the weedy species, S. latifolia harbor both seedling and adult resistance, that both are potentially costly for the host to maintain, and that the evolution of this trait in nature is molded by both the costs and the level of exposure.
_____________________________________________________________________________________________________________________________